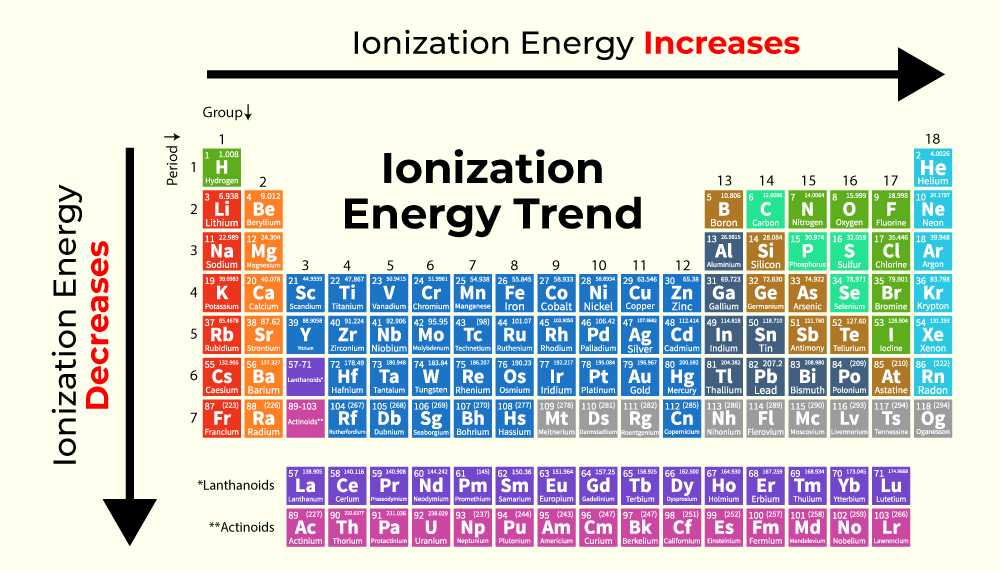
Bromine Higher Ionization Energy . First ionization energy, second ionization. You can print the list of elements by. ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period because. 123 rows these tables list values of molar ionization energies, measured in kj⋅mol −1. there are three energy levels of electrons to shield the valence electrons from the positively charged atomic nuclei. 104 rows to list the elements order by ionization energy, click on the table headers. periodic behavior is most evident for ionization energy (i), the energy required to remove an electron from a gaseous atom. for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second. This is the energy per mole necessary to. First ionization energy of bromine is 11.8138 ev. 120 rows ionization energy chart of all the elements is given below.
from www.geeksforgeeks.org
104 rows to list the elements order by ionization energy, click on the table headers. ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period because. First ionization energy, second ionization. First ionization energy of bromine is 11.8138 ev. 123 rows these tables list values of molar ionization energies, measured in kj⋅mol −1. 120 rows ionization energy chart of all the elements is given below. You can print the list of elements by. This is the energy per mole necessary to. for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second. there are three energy levels of electrons to shield the valence electrons from the positively charged atomic nuclei.
Ionization Energy Definition, Formulas, and Solved Examples
Bromine Higher Ionization Energy 120 rows ionization energy chart of all the elements is given below. for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second. 104 rows to list the elements order by ionization energy, click on the table headers. First ionization energy, second ionization. First ionization energy of bromine is 11.8138 ev. there are three energy levels of electrons to shield the valence electrons from the positively charged atomic nuclei. This is the energy per mole necessary to. ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period because. 120 rows ionization energy chart of all the elements is given below. 123 rows these tables list values of molar ionization energies, measured in kj⋅mol −1. You can print the list of elements by. periodic behavior is most evident for ionization energy (i), the energy required to remove an electron from a gaseous atom.
From techiescience.com
13 Facts on Bromine Electronegativity & Ionization Energy Bromine Higher Ionization Energy 120 rows ionization energy chart of all the elements is given below. ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period because. This is the energy per mole necessary to. 104 rows to list the elements order by ionization energy, click on the table headers. periodic. Bromine Higher Ionization Energy.
From socratic.org
Why does fluorine have a higher ionization energy than bromine? Socratic Bromine Higher Ionization Energy You can print the list of elements by. First ionization energy, second ionization. periodic behavior is most evident for ionization energy (i), the energy required to remove an electron from a gaseous atom. First ionization energy of bromine is 11.8138 ev. This is the energy per mole necessary to. there are three energy levels of electrons to shield. Bromine Higher Ionization Energy.
From exoedqopw.blob.core.windows.net
Ionization Energy Of Bromine Equation at David Taber blog Bromine Higher Ionization Energy 123 rows these tables list values of molar ionization energies, measured in kj⋅mol −1. for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second. 104 rows to list the elements order by ionization energy, click on the table headers. First ionization energy of. Bromine Higher Ionization Energy.
From periodictableguide.com
Periodic table with Ionization Energy Values (Labeled Image) Bromine Higher Ionization Energy there are three energy levels of electrons to shield the valence electrons from the positively charged atomic nuclei. This is the energy per mole necessary to. You can print the list of elements by. 120 rows ionization energy chart of all the elements is given below. First ionization energy of bromine is 11.8138 ev. First ionization energy, second. Bromine Higher Ionization Energy.
From learningschooljfreyre8d.z22.web.core.windows.net
How To Identify Ionization Energy Bromine Higher Ionization Energy periodic behavior is most evident for ionization energy (i), the energy required to remove an electron from a gaseous atom. ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period because. 104 rows to list the elements order by ionization energy, click on the table headers. there. Bromine Higher Ionization Energy.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Bromine Higher Ionization Energy You can print the list of elements by. 120 rows ionization energy chart of all the elements is given below. there are three energy levels of electrons to shield the valence electrons from the positively charged atomic nuclei. First ionization energy of bromine is 11.8138 ev. ionization energy (the energy associated with forming a cation) decreases down. Bromine Higher Ionization Energy.
From sciencenotes.org
What Is Ionization Energy? Definition and Trend Bromine Higher Ionization Energy 123 rows these tables list values of molar ionization energies, measured in kj⋅mol −1. 120 rows ionization energy chart of all the elements is given below. First ionization energy, second ionization. there are three energy levels of electrons to shield the valence electrons from the positively charged atomic nuclei. ionization energy (the energy associated with forming. Bromine Higher Ionization Energy.
From chem.libretexts.org
7.4 Ionization Energy Chemistry LibreTexts Bromine Higher Ionization Energy periodic behavior is most evident for ionization energy (i), the energy required to remove an electron from a gaseous atom. This is the energy per mole necessary to. 120 rows ionization energy chart of all the elements is given below. ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across. Bromine Higher Ionization Energy.
From exoedqopw.blob.core.windows.net
Ionization Energy Of Bromine Equation at David Taber blog Bromine Higher Ionization Energy You can print the list of elements by. 104 rows to list the elements order by ionization energy, click on the table headers. First ionization energy of bromine is 11.8138 ev. First ionization energy, second ionization. periodic behavior is most evident for ionization energy (i), the energy required to remove an electron from a gaseous atom. ionization. Bromine Higher Ionization Energy.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Bromine Higher Ionization Energy 104 rows to list the elements order by ionization energy, click on the table headers. there are three energy levels of electrons to shield the valence electrons from the positively charged atomic nuclei. This is the energy per mole necessary to. First ionization energy, second ionization. 123 rows these tables list values of molar ionization energies, measured. Bromine Higher Ionization Energy.
From www.numerade.com
SOLVED 45 of 6 Rank the following elements according to their Bromine Higher Ionization Energy there are three energy levels of electrons to shield the valence electrons from the positively charged atomic nuclei. 123 rows these tables list values of molar ionization energies, measured in kj⋅mol −1. This is the energy per mole necessary to. ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across. Bromine Higher Ionization Energy.
From ar.inspiredpencil.com
Periodic Table Ionization Energy Values Bromine Higher Ionization Energy 123 rows these tables list values of molar ionization energies, measured in kj⋅mol −1. 104 rows to list the elements order by ionization energy, click on the table headers. for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second. You can print the. Bromine Higher Ionization Energy.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Bromine Higher Ionization Energy This is the energy per mole necessary to. 120 rows ionization energy chart of all the elements is given below. there are three energy levels of electrons to shield the valence electrons from the positively charged atomic nuclei. ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period. Bromine Higher Ionization Energy.
From www.numerade.com
SOLVED Consider the elements selenium (Se) and bromine (Br). Whichhas Bromine Higher Ionization Energy ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period because. First ionization energy, second ionization. 104 rows to list the elements order by ionization energy, click on the table headers. for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom,. Bromine Higher Ionization Energy.
From exoedqopw.blob.core.windows.net
Ionization Energy Of Bromine Equation at David Taber blog Bromine Higher Ionization Energy for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second. This is the energy per mole necessary to. 123 rows these tables list values of molar ionization energies, measured in kj⋅mol −1. First ionization energy of bromine is 11.8138 ev. First ionization energy, second. Bromine Higher Ionization Energy.
From slideplayer.com
Chapter 7 Periodic Properties of the Elements ppt download Bromine Higher Ionization Energy periodic behavior is most evident for ionization energy (i), the energy required to remove an electron from a gaseous atom. You can print the list of elements by. 120 rows ionization energy chart of all the elements is given below. for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom,. Bromine Higher Ionization Energy.
From ck12.org
When we look again at the table, we can see that the ionization energy Bromine Higher Ionization Energy 120 rows ionization energy chart of all the elements is given below. there are three energy levels of electrons to shield the valence electrons from the positively charged atomic nuclei. periodic behavior is most evident for ionization energy (i), the energy required to remove an electron from a gaseous atom. First ionization energy of bromine is 11.8138. Bromine Higher Ionization Energy.
From exoedqopw.blob.core.windows.net
Ionization Energy Of Bromine Equation at David Taber blog Bromine Higher Ionization Energy First ionization energy, second ionization. there are three energy levels of electrons to shield the valence electrons from the positively charged atomic nuclei. This is the energy per mole necessary to. for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second. 123 rows. Bromine Higher Ionization Energy.